Tbilisi, Georgia – Médecins Sans Frontières (MSF), Partners In Health and Interactive Research & Development have launched a major clinical trial which seeks to revolutionise treatment for the toughest strains of tuberculosis (TB), the world’s leading infectious disease killer. The first patient started treatment in Georgia last week.
The trial is part of a UNITAID-funded transformative project called endTB, which aims to speed up and expand access to better and shorter treatments for drug-resistant forms of TB.
TB has overtaken HIV as the deadliest infectious disease, killing 1.8 million worldwide last year.
Aggravating the problem are the more complicated strains of the disease, which withstand treatments because of antibiotic resistance to the main drugs used in TB treatment.
These types of TB are known as ‘multidrug-resistant TB’ (MDR-TB) and worse, ‘extensively drug-resistant TB’ (XDR-TB). Conservative estimates from the World Health Organization (WHO) keep climbing and now place the number of yearly new cases at more than half a million.
Dr Francis Varaine, co-principal investigator of the endTB clinical trial and leader of MSF’s TB working group, says:
“Patients with MDR-TB often fear the drugs as much as the disease. They lose their energy, perhaps their hearing or eyesight, and put their lives on hold for up to two years.”
He adds: “Having followed MSF TB projects around the world for many years, I am convinced that the recent approval of two new drugs, bedaquiline and delamanid, represents an unique opportunity that should not be missed to develop new treatment regimens that could be life changing for MDR-TB patients. While both drugs have shown very promising results when added to the standard, long and badly-tolerated MDR-TB treatments, we know little about how to optimise them.
“Without further research, we are only scratching the surface and patients continue to suffer.”

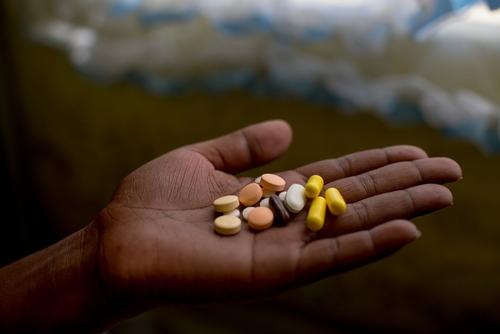
Current treatments for MDR-TB are long (up to 24 months), ineffective (only 50 per cent succeed) and often cause terrible side effects, including acute psychosis and permanent deafness. Patients endure months of painful, daily injections and ingest up to 14,000 pills. Moreover, the costliness, difficulty and length of current treatments make them hard to implement in many high-burden countries.
To address the problem, this phase III clinical trial uses the first TB drugs developed in almost 50 years — bedaquiline and delamanid — to find radically shorter (nine months), injection-free, more tolerable treatments for MDR-TB.
The new drugs will be combined into experimental new treatments with other oral TB drugs such as clofazimine, linezolid, fluoroquinolones and pyrazinamide.
Today only about 10 per cent of MDR-TB sufferers worldwide can hope to access successful treatment, and less than two per cent can access bedaquiline or delamanid.
The clinical trial will expand the evidence base for using bedaquiline and delamanid, particularly as part of simpler and better treatments that would be easier to implement in resource-poor countries with significant TB burdens. Evidence of success of these regimens, coupled with support for their implementation, would dramatically expand access to successful treatment.
The endTB project is expected to enroll an estimated 2600 MDR-TB patients on treatment with the new TB drugs and 750 patients will take part in a clinical trial across six countries: Georgia, Kazakhstan, Kyrgyzstan, Lesotho, Peru and South Africa. These are all countries with significant TB burdens, where endTB partners support local MDR-TB treatment activities.
The trial benefits from the expertise of Harvard Medical School, Epicentre and the Institute of Tropical Medicine Antwerp. The clinical trial team also works closely with Ministries of Health and National Tuberculosis Programs (NTP) in each country, as well as the WHO and ethics review boards.
This is the second TB clinical trial sponsored by MSF. The first, TB PRACTECAL, launched in Uzbekistan in January 2017.