1. General Commitment
As a medical organisation, MSF regularly dispenses drugs to patients in the programmes it runs all over the world.
MSF attaches the utmost importance to the quality of the medicines that are given/dispensed to patients.
MSF strongly considers that any patient wherever he/she lives has a right to be treated with effective and safe drugs.
2. MSF drugs procurement chain
Like many other humanitarian actors, MSF is aware that the quality of drugs is not always properly guaranteed on the global market.
The general policy of the organisation is to select its sources of pharmaceuticals and supply them to its teams wherever they operate and wherever MSF is authorised to do so.
To this end, MSF has set up its own European Supply Centres (MSF Logistique in Bordeaux, France in 1986; MSF Supply in Neder-Over-Heembeek, Belgium in 1989; and Amsterdam Procurement Unit in Amsterdam, The Netherlands in 1984). The objective is twofold:
- Operational: to supply medical and non-medical relief goods to MSF teams according to the requested delivery date.
- Qualitative: to keep a strict control on the quality of supply and make sure that it is compliant with MSF's technical requirements.
When drugs are supplied by the European Supply Centres, MSF considers that it is the moral responsibility of the organisation to guarantee their quality. The MSF Executive Committee has consequently asked MSF pharmacists to develop a qualification procedure in order to achieve this objective. The acceptability of pharmaceutical products intended for import by MSF in countries for its programmes, and supplied by MSF European Supply Centres, is assessed according to the "MSF Qualification Scheme" for International Pharmaceutical Supply.
In some countries, however, MSF is not authorised to import medicines. In these cases, the MSF qualification procedure cannot apply and the responsibility to assure the quality of the drugs rests with national drug authorities.
3. Definition
The qualification of the medicines is an essential part of the quality assurance. It verifies that the products meet at least the norms and standards set by international organizationsWHO Pre-Qualification Programme
4. Objective
To identify and qualify pharmaceutical products that are compliant with the MSF quality requirements.
5. The Evaluators
The evaluation work is essentially carried out by MSF Headquarters Pharmacists based in the European Supply Centres, the Access Campaign, and the International Office, under the coordination of the International Pharmacist Coordination. External experts are consulted and/or contracted for specific parts of the evaluation (e.g. Good Manufacturing Practice (GMP) audits, Active Pharmaceutical Ingredients specifications, bio equivalence studies, etc.)
6. Principles
The MSF qualification procedure is based on a principle of mutual trust. The manufacturer is asked to certify the veracity and accuracy of the information and documents submitted to MSF.
Mistakes or omissions (intentional or not) may lead to the disqualification of the product and/or the manufacturer.
The MSF Qualification Scheme is neither intended to interfere with the WHO pre-qualification initiative, nor to duplicate any existing work (GMP inspections, product evaluations) carried out by stringent Drug Regulatory Authorities.
Therefore,
- Any WHO pre-qualified product that is included in the MSF List of Medicines is automatically qualified by MSF
- Any product registered in a highly regulated country that is included in the MSF List of Medicines, is automatically qualified by MSF.
- Any other product should be qualified through the qualification process described below.
MSF is not a Medicines Regulatory Agency.
The MSF Qualification Scheme has been exclusively designed for the organisation and the decisions taken are only valid for MSF.
The MSF Qualification Scheme is based on internationally recognised principles and built on two pillars:
- The manufacturing site GMP assessment
- The pharmaceutical product dossier assessment
The norms and standards that underline the MSF Quality System are:
- Those published by the WHO (GMP guidelines, Technical Report Series)
- The WHO International Pharmacopoeia, the European Pharmacopoeia, The British Pharmacopoeia and the United States Pharmacopoeia
- The MSF specifications for pharmaceutical products, based on international standards
The MSF qualification procedure is open to any manufacturer regardless of the country of operation.
7. Steps in the qualification procedure
- Expression of interest;
- Screening and evaluation of Manufacturer Information File;
- GMP Assessment of the manufacturing site (through an audit or a technical visit);
- Final decision on the evaluation of the manufacturing site(s);
- Request and submission of pharmaceutical product dossier;
- Evaluation of pharmaceutical product dossier;
- Result of the evaluation of the product dossier (product-manufacturer couple qualified or not).
- Monitoring and follow-up
7.1. Expression of interest
Any manufacturer who wishes to participate to the MSF qualification procedure is invited to express their interest by filling in the "Manufacturer Information File" (MIF).
The manufacturer should send the MIF and requested annexes to the MSF International Office, by e-mail or by post using the following address:
Assistant to QA coordinators
Rue de l’Arbre Bénit 46
1050 Brussels
Belgium
An acknowledgement of receipt will be sent in return to the manufacturer.
7.2. Screening and evaluation of the Manufacturer Information File
Each file submitted by a manufacturer is first screened for completeness.
Special attention will be paid to the list of products submitted by the manufacturer as an annex to the MIF. The evaluation procedure will only be initiated for manufacturers who produce pharmaceuticals that match with MSF interests.
7.3. GMP Assessment of the manufacturing site
7.3.1. Technical visit of the manufacturing site
GMP inspections carried out by the WHO PQ inspectors, or Inspectorates recognised by MSF are taken into consideration by MSF.
A technical visit occurs when sites already found GMP compliant after a WHO PQ or an MSF recognised inspectorate inspection are visited by a MSF HQ pharmacist.
A "technical visit" is not a full GMP audit. It is usually shorter and designed to make sure that the products of potential interest for MSF are actually manufactured on the approved premises.
7.3.2. Audit of the manufacturing site
For facilities that have not been previously inspected and approved by WHO PQ or a SRA inspectorate, the MSF International Pharmacists Coordinator will appoint a GMP expert to perform an audit. Audits are carried out against the WHO GMP guidelines.
7.4.3. Schedule of audits and technical visits
The MSF International Pharmacists Coordinator will inform the manufacturer whenever an audit or a technical visit of the plant is needed. Taking into account the constraints of the manufacturer and those of MSF, the audit or technical visit will be scheduled at the shortest possible notice (whatever that may be).
7.3.4. Reporting
The auditor writes a report on the findings of the audit that is sent to the manufacturer.
If any additional information is required or corrective action has to be taken by the manufacturer, MSF pharmacists will postpone their final conclusions until the additional information and corrective actions have been evaluated by the auditor and/or the MSF pharmacists.
7.4. Final decision on the evaluation of the manufacturing site
On the basis of the conclusions of the technical visit or audit, the MSF International Pharmacists Coordinator will inform the manufacturer in writing of the outcome of the evaluation which can be:
- The site is approved by MSF
- The site is NOT approved by MSF
- A new audit is needed. In this case, a tentative schedule is proposed to the manufacturer. The decision to approve (or not) the manufacturing site is postponed until the outcome of the re-audit has been evaluated by the MSF pharmacists.
7.5. Request and submission of pharmaceutical product dossier
Manufacturers with at least one MSF approved manufacturing site will be asked to submit detailed technical information on the products that present an interest to the organisation. The MSF pharmacists will provide the approved manufacturer with an electronic formatted copy of the Inter Agency Product Questionnaire (IAPQ).
The IAPQ is a tool that has been developed by the MSF pharmacists in collaboration with the UNICEF Supply Division, the WHO Procurement Department, WHO Pre-Qualification Programme, the International Committee of the Red Cross, The Global Fund and UNFPA.
The approved manufacturer will submit the questionnaire to MSF together with the requested annexes and a sample of the product concerned. All the information should be submitted in English.
Questionnaires that are not complete will not be considered for evaluation. In this case, the manufacturer will be informed that the questionnaire is incomplete and will be given a deadline to complete it. If the manufacturer is not able to submit the complementary information within the given schedule, the product dossier will be rejected.
7.6. Evaluation of the pharmaceutical product dossier
Key indicators of the quality assurance of a product are assessed:
- Status of registration by National Drug Regulatory Authority (NDRA) in the country of origin
- Active Pharmaceutical Ingredient (API) Quality Assurance
- Finished Product (FP) specifications
- Stability studies on the finished products
- Sample (packaging and labelling)
- Manufacturing site WHO GMP level of compliance
- Therapeutic equivalence (if needed)
A rating is given for the first six indicators.
This rating reflects the level of compliance (1 = poorly compliant, 6 = fully compliant) of each indicator with the MSF standards and specifications.
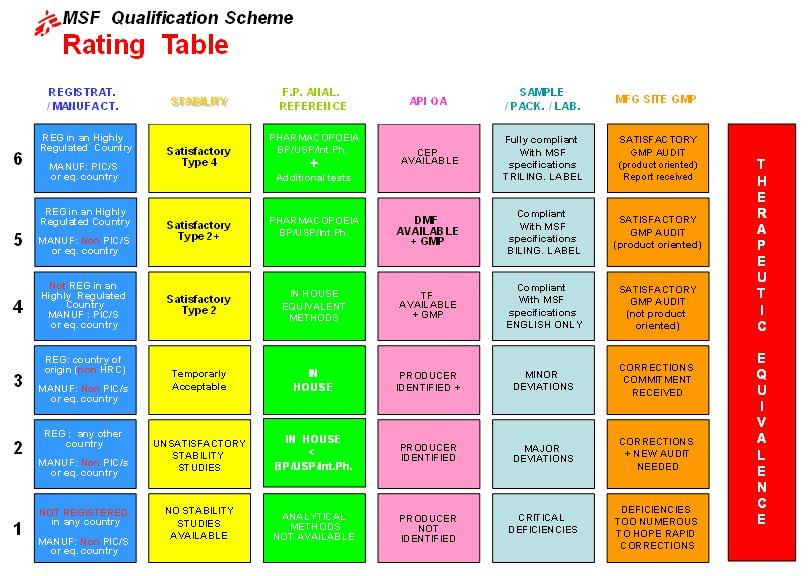
Any rating lower than three means that the product cannot be qualified for MSF.
Therapeutic equivalence
A similar rating is not applicable to this important indicator. The WHO clearly states that "reasonable assurance must be provided that the multi-source product is therapeutically equivalent and interchangeable with the comparator product" (WHO Technical Report Series 937, annex 7).
In the same document, WHO experts add that "bioequivalence is significant only if compliance with Good Manufacturing Practices and sourcing of Active Pharmaceutical Ingredients are well controlled".
MSF also considers that an evaluation of the therapeutic equivalence only makes sense if, and only if, the other parts of the product file have already been satisfactorily assessed by the MSF pharmacists.
The need for in vivo equivalence studies or the qualification for a biowaiver is evaluated for each product on the basis on the following WHO documents:
- "Guidelines on registration requirements to establish interchangeability"
- "Proposal to waive in vivo bioequivalence requirements for WHO Model List of Essential Medicines immediate release, solid oral dosage forms"
(WHO Technical Report Series 937, annexes 7 and 8)
At the time of the submission of the product file, the manufacturer should provide proof of therapeutic equivalence.
7.7. Result of the evaluation of the product dossier
The International Pharmacists Coordination will do the final review of the product dossier and decide about the outcome of the evaluation:
- The product is compliant with the MSF requirements and specifications
- The product is NOT compliant
In the first case:
- The pharmacist in charge of the dossier informs the manufacturer in writing that the product is approved by MSF
- The details of the approved product are summarised in a Product Specification Sheet (PSS) that is sent for signature to the manufacturer as a commitment to supply MSF with a product according to the agreed specifications
- It is only after reception of the signed PSS that the product is considered to be "MSF qualified"
- Following the qualification of a product, the commercial negotiation can start with the European Supply Centres
In the second case:
The manufacturer is informed that its product does not meet the MSF requirements and specifications and therefore cannot be approved.
7.8. Monitoring and follow up
MSF site approvals are granted for a period of 3 years.
Periodic re-audits or new technical visits are scheduled accordingly.
Recent satisfactory WHO PQ/SRAs inspections can be taken into consideration for the renewal of a GMP approval.
Monitoring visits can also be planned in order to trace back the records of batches that have been delivered to MSF European Supply Centres.
The MSF qualification of a product is valid for a period of 5 years.
During the period of validity of a qualification, the manufacturer is asked to advise MSF promptly of any changes in the manufacture or specifications of the product. Details of the change are declared in a variation form which the manufacturer is requested to complete.
The implementation of the changes is subject to prior formal approval by MSF. If MSF accepts the proposed variation, a new PSS will be sent for signature to the manufacturer.
If MSF does not accept the proposed variation, the product will be disqualified.
Only products that are conforming to the signed PSS can be supplied to MSF European Supply Centres
The implementation of a change (in the manufacturing process or the specifications of a MSF qualified product) that has not been approved beforehand by MSF will be considered as a breach of contract.
8. Confidentiality commitment
As a principle, all the information communicated to MSF in the context of the qualification process is considered confidential and will be treated as such.
Any person involved in the assessment process is asked to sign a confidentiality agreement by which he/she ensures that:
- The confidential information is not used for any purpose other than the evaluation activities conducted for MSF
- Confidential information is not disclosed or provided to any person who is not bound by similar obligations of confidentiality
- All information collected, submitted or observed during the evaluation process is the property of MSF
Manufacturers are reminded that all information contained in the Inter Agency Product Questionnaire may be shared with the UNICEF Supply Division, the WHO Procurement Department, WHO Pre-Qualification Programme, the International Committee of the Red Cross, The Global Fund and UNFPA.
9. Commercial negotiation
Only manufacturers with at least one MSF approved manufacturing site will be allowed to participate in commercial negotiations. Only MSF qualified products will be purchased by MSF European Supply Centres.
10. Conflict of interest
All personnel involved in the MSF qualification procedure are asked to sign a "Confidential Agreement" by which they confirm that no situation of real, potential or apparent conflict of interest is known to them, including that they have no financial or other interest in, and/or relationship with a party.



