13
13
21
21
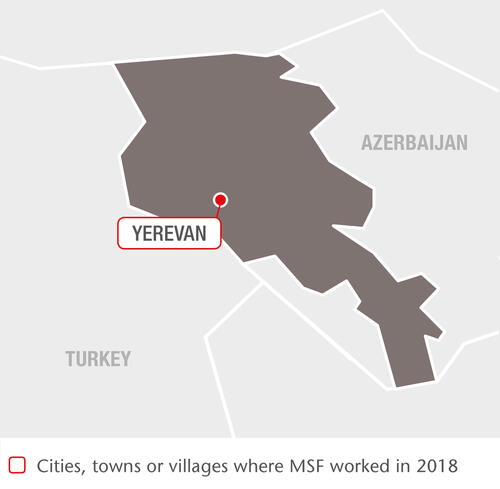
Over the last 14 years, we have progressively expanded our programme so that the latest treatment for drug-resistant TB (DR-TB) is now available throughout Armenia. Since 2015, we have been participating in the endTB project, an international initiative aimed at finding shorter, less toxic and more effective treatments for DR-TB.
Enrolments in the observational study were completed in Armenia in June 2017, with a total of 106 patients participating. Follow-up continued throughout 2018 and the project is scheduled for completion in March 2019.
We have also been offering hepatitis C treatment to patients with DR-TB and chronic active hepatitis C since 2016: 20 per cent of DR-TB patients followed by our teams at that time were co-infected with hepatitis C, which can affect liver function and exacerbate the side effects of DR-TB treatment.
In 2018, we started an epidemiological study aimed at documenting the safety and efficacy of combining the newest treatments for DR-TB with direct-acting antivirals for hepatitis C. In April, we also sent a team to perform thoracic surgery on six TB patients in Armenia, where such treatment is not otherwise available.
On completion of the endTB project and our epidemiological study in March 2019, we will hand over our remaining activities in Armenia to the national health authorities. In preparation, in 2018 our teams started providing theoretical and on-the-job training to doctors and nurses working in TB clinics across the country, with case management already handed over in many areas by the end of 2018.