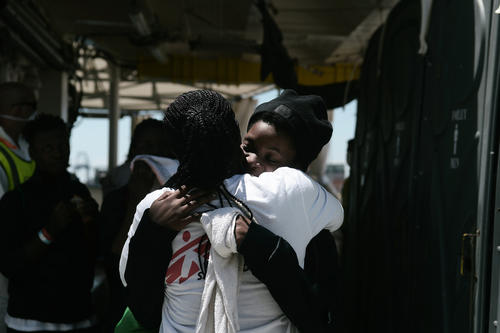2,220
2,22
660
66
810
81
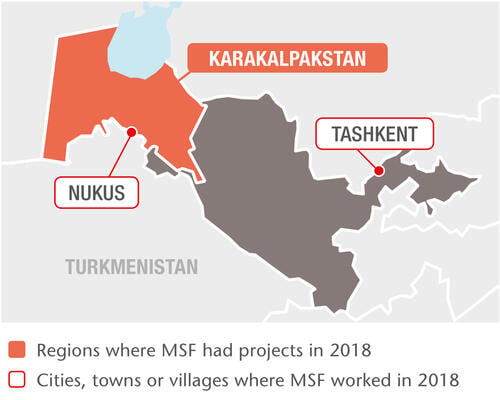
Our TB project in Nukus, the capital of the Republic of Karakalpakstan, has two components: comprehensive patient-centred care and clinical research into shorter, more tolerable and more effective treatments. Our approach includes a shorter, nine-month treatment regimen and home-based outpatient care.
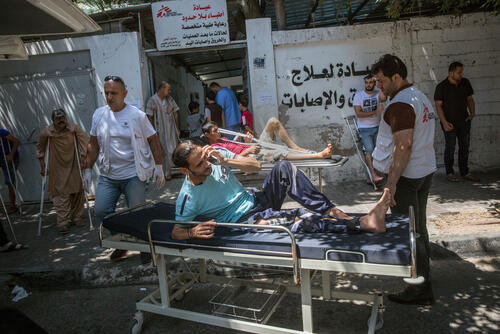
In 2018, we started 2,220 patients on TB treatment in Uzbekistan. Of these, 660 were drug-resistant in some form, including 450 multidrug-resistant and 70 extensively drug-resistant cases; 199 were treated with new or repurposed drugs. We are supporting the rollout of World Health Organization treatment recommendations throughout Karakalpakstan, where we also manage a state-of-the-art laboratory equipped with some of the most advanced diagnostic instruments.
We launched the multi-site TB PRACTECAL clinical trial in Nukus in 2017 to evaluate the effectiveness of two of the newest TB drugs – bedaquiline and pretomanid – on a much shorter regimen of just six months. By the end of 2018, the Nukus site had recruited 104 patients and an additional site in Tashkent had been approved to start recruitment in early 2019.
We also work with the Ministry of Health to care for people living with HIV. Based in Tashkent since 2013, our project focuses on integrated care for HIV patients co-infected with either hepatitis C, syphilis, or other sexually transmitted infections. In 2018, we began working in clinics that serve at-risk groups such as sex workers, people who inject drugs and men who have sex with men. In 2018, we initiated 750 people on hepatitis C treatment and 810 on antiretroviral (ARV) therapy for HIV.